NeuroLog System Application Note
Juxtacellular Labelling & Extracellular Recording with the NL102G DC Amplifier

What is juxtacellular labelling?
Juxtacellular labelling is a technique that allows extracellular electrophysiological identification and investigation of individual central nervous system neurons, with subsequent morphological visualisation of their cell body and dendritic architecture. Juxtacellular labelling was pioneered by Pinault (1996 & 2011) and is widely used for in vivo and in vitro labelling of a variety of cell types.
Briefly, the technique involves micro-manipulator controlled positioning of a label filled glass microelectrode within close proximity of a neuron of interest. Once a suitable cell is located, extracellular electrophysiological and/or pharmacological studies are carried out. Upon completion, labelling is achieved by “tickling” the cell with repetitive low amplitude pulses of current which cause the labelling agent to enter the cell via micro-electroporation of the cell membrane. The tissue may then be fixed and sectioned prior to microscopic visualisation of the labelled cells.
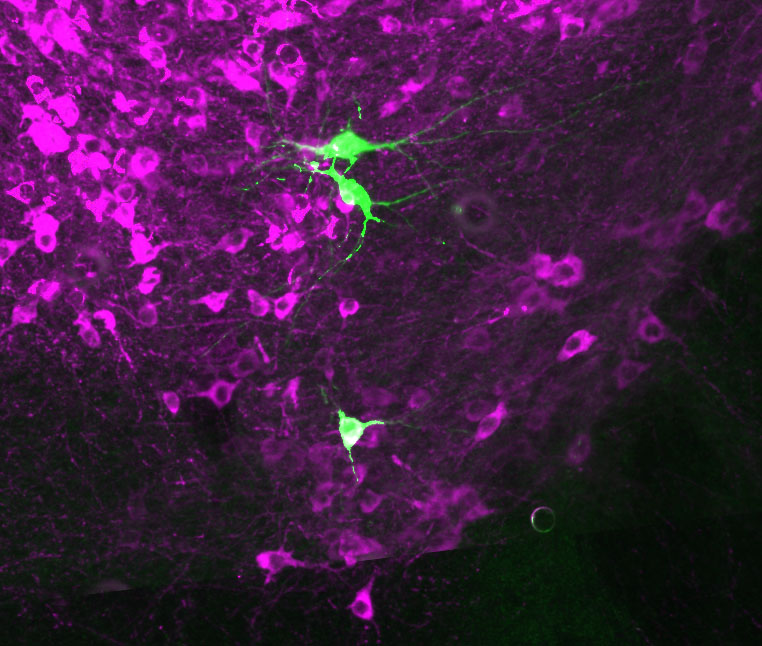
Juxtacellular labelling and an examination of Nitric Oxide mediated transmission
Dr Sasha Gartside, a senior lecturer based at the University of Newcastle, recently published a paper in the European Journal of Neuroscience (Gartside et al., 2020) which examined the role of nitric oxide in 5-HT (serotonin) neurons of the midbrain raphe nuclei. Dr Gartside and her colleagues employed the Digitimer NeuroLog System to record from and then juxtacellularly label these neurons. These studies supported the proposal that Nitric Oxide acts as a trans-synaptic and autocrine signaller in 5-HT neurons within dorsal raphe and midbrain raphe nuclei.
Here we discuss some of the technical aspects of juxtacellular labelling with reference to the methods published by Dr Gartside and her colleagues. Digitimer is extremely grateful to Dr Gartside for sharing experimental details, screenshots from CED Spike2 software and an image of a labelled neuron, that are used in this article.
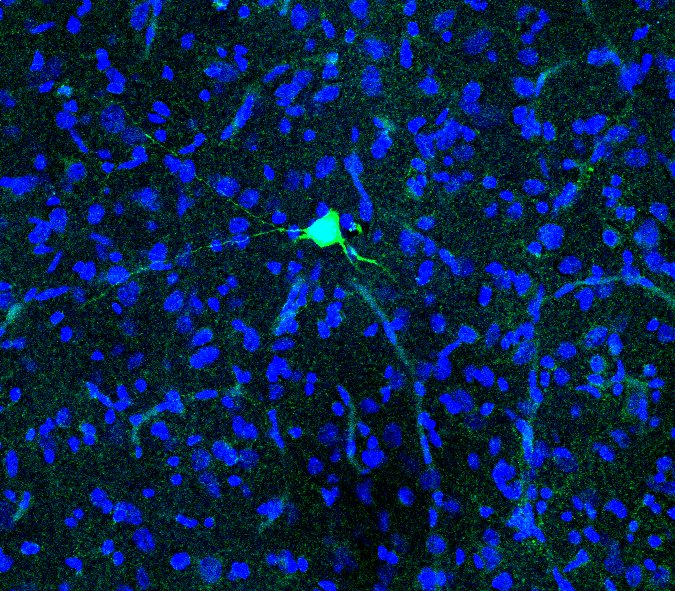
Juxtacellular labelling requires a firm, straight microelectrodes
Successful juxtacellular labelling depends on an ability to produce glass microelectrodes with the correct shape and tip specifications. Gartside et al. used the Narishige PE-2 vertical puller, which has now been superseded by the Narishige PE-22, available from Digitimer. Both the PE-2 and Narishige PE-22 puller use a combination of magnetic force and gravity to produce the type of firm, straight microelectrodes ideal for juxtacellular labelling. Microelectrodes with an impedance of 5-10Mohms are filled saline with 0.5%-1% biocytin or neurobiotin.

What sort of extracellular amplifier do I need?
In terms of amplifiers, any microelectrode bridge amplifier will be suitable, but it must have current injection capabilities, as these are used to modulate the firing of and label the cell of interest. Our NeuroLog System NL102G DC Preamplifier has become a firm favourite amongst exponents of the juxtacellular labelling technique.

Starting with the NL102G DC Preamplifier, the Digitimer NeuroLog System is easily configured to (i) make extracellular single unit recordings (ii) provide an audible indication of firing frequency and (iii) label cells following electrophysiological and pharmacological investigation:-
- NL102G DC Preamplifier – Although originally designed for intracellular recording, the NeuroLog System NL102G DC Amplifier is popular among researchers carrying out juxtacellular labelling. When used with our NL111 Electrode Holders the remote NL102GH headstage is compatible with glass-filled microelectrodes. Electrode impedances can be monitored using the Impedance Check function, although this is also accomplished via the Bridge Balance controls. Currents of up to ±100nA can be passed by the NL102G, with amplitude adjusted by a 10-turn dial. Current pulse timing is controlled by a TTL GATE IN socket, allowing a computer-based DAQ system and software or other NeuroLog System modules to activate repetitive current injections. It is even possible to use a ±10V signal applied to the EXT STIM IN socket to modulate current amplitude.
- NL106 AC/DC Amplifier – As the NL102G was designed for intracellular recording, it has a relatively low maximum gain, so the NL106 is used to boost this by up to x100.
- NL125/6 Band-pass Filter – The output from the NL102G is DC coupled, so addition of the NL125/6 allows low and high cut filters to be applied.
- NL120S Audio Amplifier and NL985S Speaker – The NL120S can be used to convert the single unit activity into audible “pops”, allowing electrophysiologists to monitor the cell’s behaviour without taking their attention from the microscope.
Note that NeuroLog System modules need to be installed in our NL900D Rack and Power Supply Unit and there would be a requirement for several cables to connect between the DAQ hardware and the NeuroLog System.
Identifying a target neuron and recording its activity
Under microscopic visualisation and with precise manipulator control, the microelectrode tip needs to be manoeuvred into position as close as possible to the cell of interest. Spontaneous electrical activity may be monitored visually via the data acquisition software (e.g. CED Spike2), but it is also helpful to convert single unit action potentials into an audible signal that can be listened to while the microelectrode is moved. In the audio file you can hear the action potential firing as distinct “clicks”.
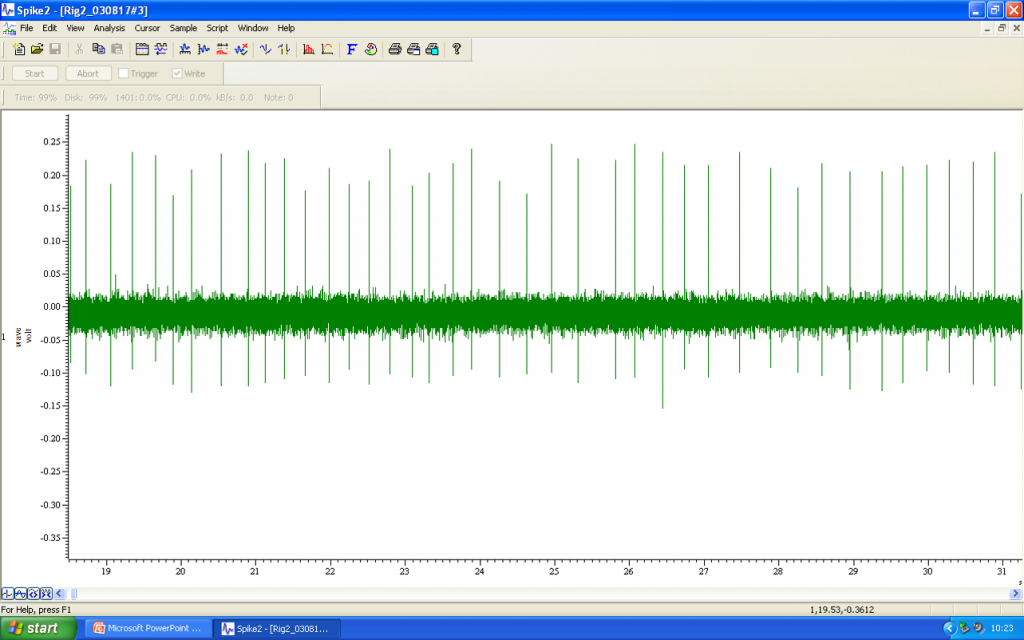
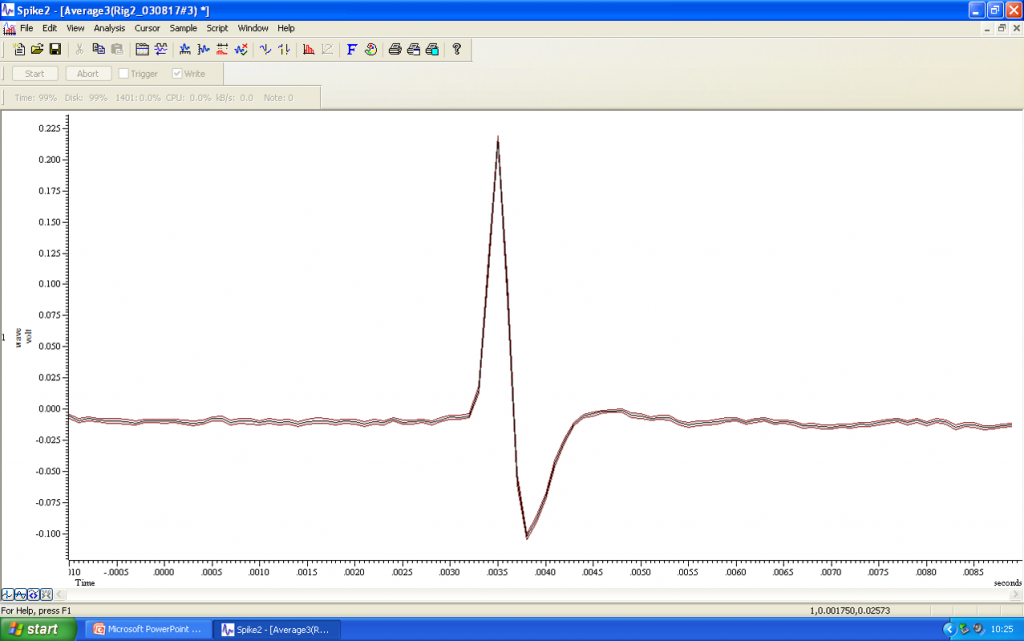
Using current pulses to juxtacellularly label a neuron
Once electrophysiological or pharmacological studies are complete, the next step involves the juxtacellular labelling of the cell that has been investigated. While continuing to monitor the firing activity of the neuron, low amplitude +ve current pulses of 200ms duration are applied through the recording electrode every 400ms.
The amplitude of these pulses is gradually increased from 10’s of microamps to a level of 1.2mA. Spontaneous firing should continue as the current amplitude increases, but entrainment will follow, with the neuron producing volleys of action potentials during the depolarisation phase of the current injection. In the audio file, you can hear the “clicks” that correspond to the action potentials, as well as a “shushing” noise of the current injections.
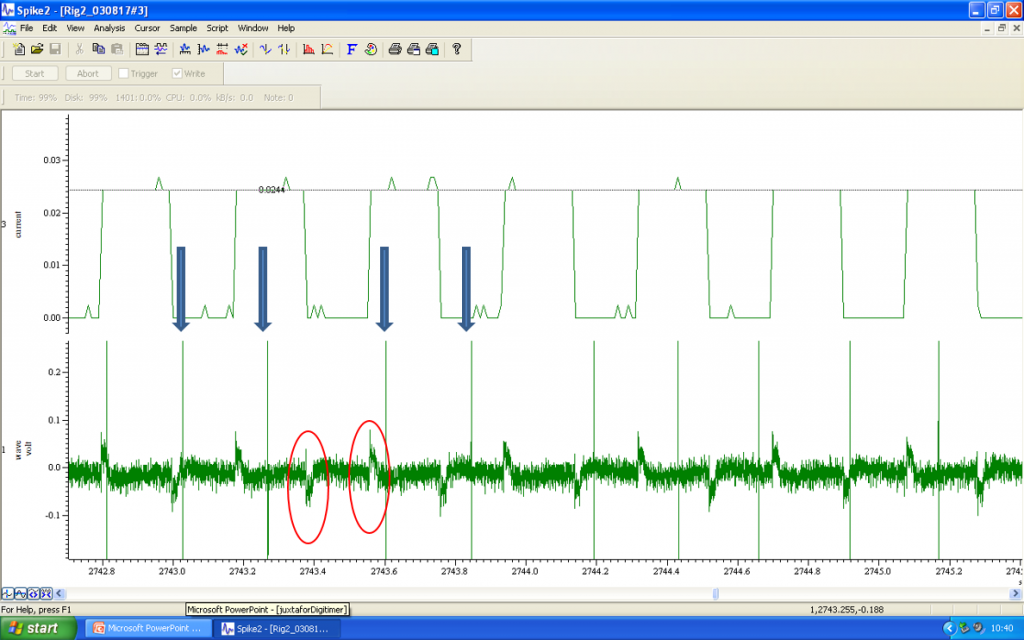
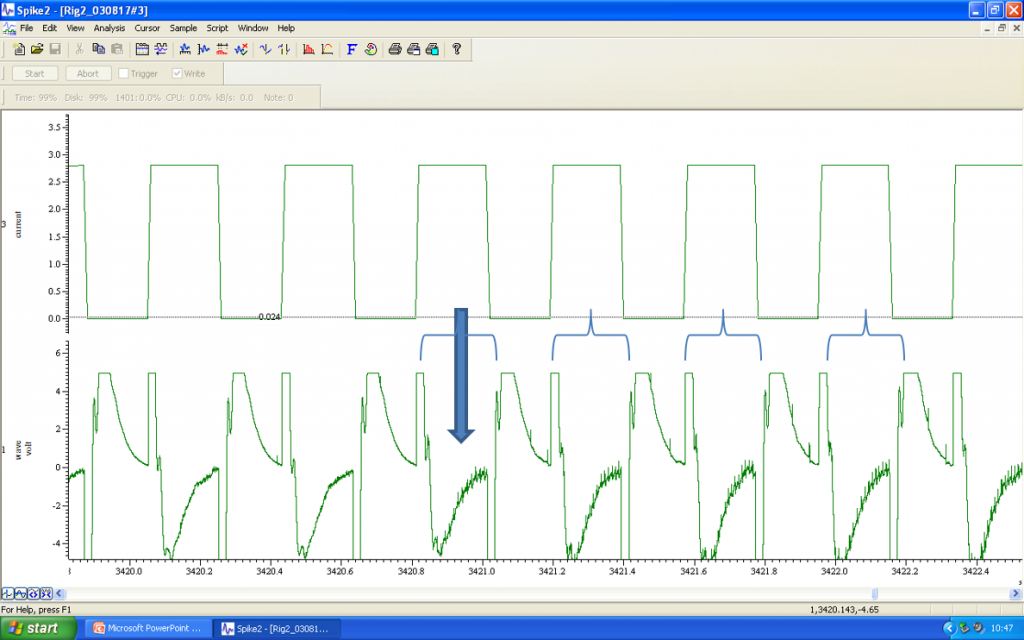
Current injections are repetitively applied for several minutes, or as long as possible, leading to iontophoretic labelling of the cell of interest. Once an adequate number of cells have been labelled within a single slice, the tissue can be extracted and fixed for subsequent immuno-labelling in parallel with visualisation of the neurobiotin/biocytin filled cells.
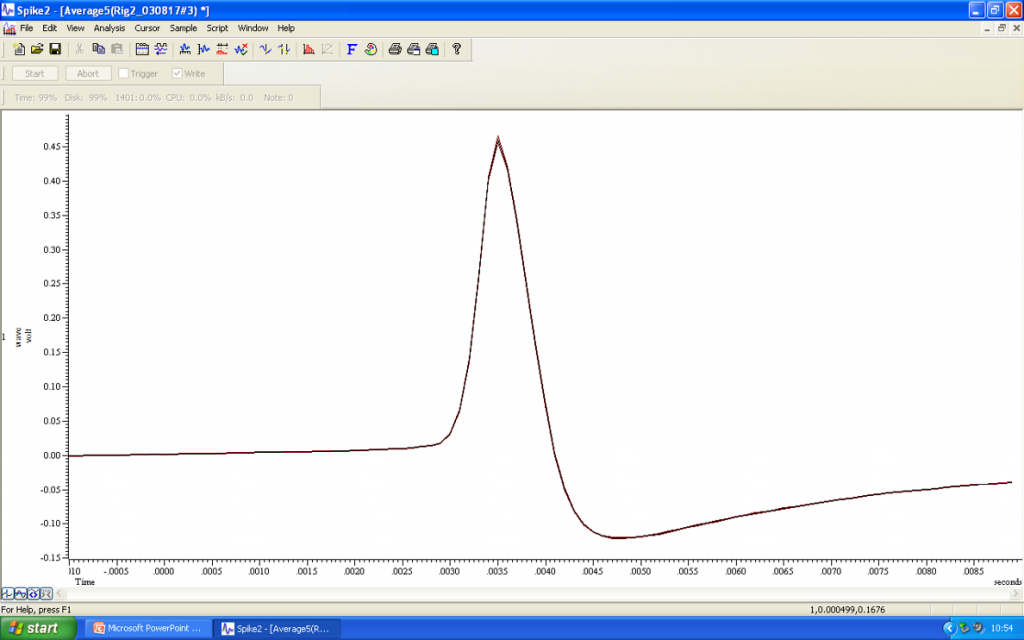
Want to find out more?
This brief article provides a very superficial overview of the technique, but if you would like to learn more about juxtacellular recording, we recommend the papers below as a good starting point. For more information about the NL102G DC Preamplifier, the NeuroLog System or other complementary products we can supply, please get in touch with Digitimer.
References
, , , , . (2019) Opposite responses to aversive stimuli in lateral habenula neurons. Eur J Neurosci.; 50: 2921– 2930. https://doi.org/10.1111/ejn.14400
, , , et al. (2020) A role for nitric oxide in serotonin neurons of the midbrain raphe nuclei. Eur J Neurosci.; 51: 1881– 1899. https://doi.org/10.1111/ejn.14713
Pinault, D. (1996) A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin, Journal of Neuroscience Methods, Volume 65, Issue 2, 1996, 113-136, ISSN 0165-0270, https://doi.org/10.1016/0165-0270(95)00144-1.
Pinault D. (2011) The Juxtacellular Recording-Labeling Technique. In: Vertes R., Stackman Jr. R. (eds) Electrophysiological Recording Techniques. Neuromethods, vol 54. Humana, Totowa, NJ. https://doi.org/10.1007/978-1-60327-202-5_3
Boris Mlinar, Alberto Montalbano, Gilda Baccini, Francesca Tatini, Rolando Berlinguer Palmini, Renato Corradetti (2015) Nonexocytotic serotonin release tonically suppresses serotonergic neuron activity. J Gen Physiol; 145 (3): 225–251. doi: https://doi.org/10.1085/jgp.201411330
Schweimer, J.V., Coullon, G.S.L., Betts, J.F., Burnet, P.W.J., Engle, S.J., Brandon, N.J., Harrison, P.J. and Sharp, T. (2014), Increased burst-firing of ventral tegmental area dopaminergic neurons in d-amino acid oxidase knockout mice in vivo. Eur J Neurosci, 40: 2999-3009. https://doi.org/10.1111/ejn.12667


