Product Focus
DG2A Train/Delay Generator – A Simple Solution to Repetitive Stimulus Triggering
Overview
The DG2A is a compact, free-standing and battery powered instrument which can be used to generate TTL compatible trigger pulses required for repetitive stimulation. Researchers acquiring data using a digital acquisition interface often have the capability to generate digital trigger pulses from within their acquisition software, but what do you use if you dont have such hardware?
For situations where a DAQ interface is not used or where a system needs to be more portable, the DG2A provides the perfect solution. As a result, the DG2A is commonly employed to trigger our DS7A/AH and DS7R high voltage constant current stimulators as well as our DS2A and DS3 voltage and current isolators. Featuring DELAY controls, it is also useful for determining nerve or axonal Effective Refractory Period (ERP) through the production of a delayed second pulse or for examination of paired pulse potentiation.
Various operating modes allow output pulses to be produced singularly (SINGLE), continuously (FREE-RUN & GATED) or in a burst (TRAIN), with the burst/train duration and pulse frequency determined by the front panel controls. In each of the modes (except FREE-RUN), outputs can be initiated either by the front panel push button, a TTL compatible trigger/gating pulse or a suitable foot switch.
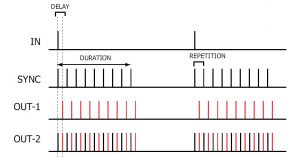
 The DG2A features control of train duration over three full decades, pulse repetition rate (or frequency) within that train over five decades and control of the delayed pulse over three decades. It has two BNC output sockets:-
The DG2A features control of train duration over three full decades, pulse repetition rate (or frequency) within that train over five decades and control of the delayed pulse over three decades. It has two BNC output sockets:-
(i) the SYNC output produces a pulse to trigger recording devices or synchronise other equipment.
(ii) the OUT output produces either a delayed version of the same or by toggle switch selection, pairs of delayed and non-delayed pulses (as would be necessary for ERP studies).
[su_youtube url=”https://www.youtube.com/watch?v=wYLoZO9YgjI”]
The unit is especially suitable for use with our DS2A Isolated Constant Voltage and DS3 Isolated Current Stimulators which have their own Pulse Duration controls. A mounting frame (part number D121-11) is available so that two units of either DG2A, DS2A or DS3 can be mounted in 19” rack. The instrument is powered by an internal 9V battery (PP3 – 6R61 style).
 Operating Modes
Operating Modes
TRAIN Mode
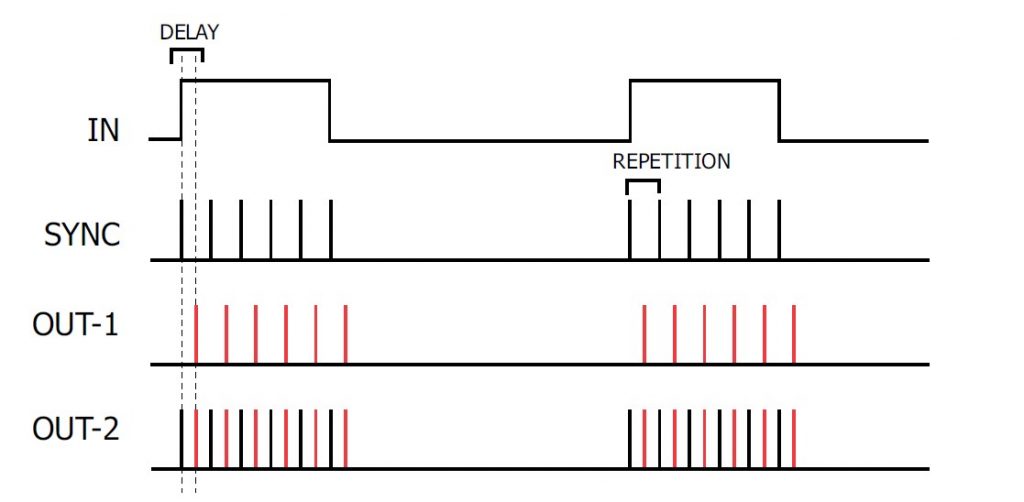
In TRAIN mode, a regular button press or TTL compatible trigger received at the IN socket is translated into a SYNC pulse train with a pulse repetition rate and train duration determined by the REPETITION and DURATION settings. The DELAY dial allows the introduction of a delayed pulse (indicated in RED) after each SYNC pulse which can be detected at the OUT-1/OUT-2 output.
GATED Mode
In GATED mode, the DG2A will output a train of pulses at the SYNC socket while the input at the INsocket is TTL high. This allows the operator to GATE the train of pulses on and off with an external device. The pulse repetition rate is determined by the REPETITION setting. The DURATION control has no function in this mode. The DELAY dial allows the introduction of a delayed pulse (indicated in RED) after each SYNC pulse, which can be detected at the OUT-1/OUT-2 output.
 FREE-RUN Mode
FREE-RUN Mode
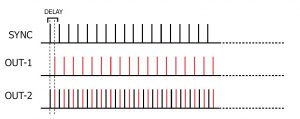
In FREE-RUN mode, the DG2A will continuously output SYNC pulses with a pulse repetition rate determined by the REPETITION setting while the unit is ON. The DURATION control has no function in this mode. The DELAY dial allows the introduction of a delayed pulse (indicated in RED) after each SYNC pulse which can be detected at the OUT-1/OUT-2 output.
SINGLE Mode
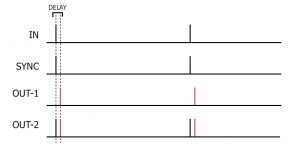
In SINGLE mode, a single TTL compatible trigger received at the IN socket or push button press is translated into a single SYNC pulse. The REPETITION and DURATION controls have no function in this mode. The DELAY dial allows the introduction of a delayed pulse (indicated in RED) after each SYNC pulse which can be detected at the OUT-1/OUT-2 output.
So where has the DG2A been used?
In 2013 Jonas Saugy and colleagues investigated the physiological effects of the most challenging mountain ultra-marathon (MUM) in the world: a 330-km trail run with 24000 m of positive and negative elevation change. Neuromuscular fatigue (NMF) was assessed before, during and after the MUM in experienced ultra-marathon runners and in a control group with a similar level of sleep deprivation.
As part of their research, they employed our DS7AH stimulator to make force and EMG measurements. Maximal voluntary contraction (MVC) force loss of Knee Extensor (KE) and Plantar Flexor (PF ) muscles was evaluated to provide an index of global fatigue. The voluntary activation ratio of KE and PF was assessed using superimposed high-frequency (100 Hz) doublet triggered via the Digitimer DG2A to detect central fatigue.
The paper was published in PloS ONE and is now the most highly cited DG2A paper at 116 citations (until May 2021).
Saugy J, Place N, Millet GY, Degache F, Schena F, et al. (2013) Alterations of Neuromuscular Function after the World’s Most Challenging Mountain UltraMarathon. PLoS ONE 8(6): e65596.
https://doi.org/10.1371/journal.pone.0065596
The DG2A’s Moment of Television Fame
The DG2A and our DS7A stimulator made a brief appearance on the BBC’s “Trust me I’m a Doctor” when the presenter, Dr Michael Mosley used them to induce cramp in his arm.
Recent DG2A Publications
Cook, D. N., Thompson, S., Stomberg-Firestein, S., Bikson, M., George, M. S., Jenkins, D. D., & Badran, B. W. (2020). Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation. Brain Stimulation, 13(3), 800–803. https://doi.org/10.1016/j.brs.2020.02.028
Cramer, J. V., Gesierich, B., Roth, S., Dichgans, M., Düring, M., & Liesz, A. (2018). In vivo widefield calcium imaging of the mouse cortex for analysis of network connectivity in health and brain disease. BioRxiv. https://doi.org/10.1101/459941
Dundon, N. M., Shapiro, A. D., Babenko, V., Okafor, G. N., & Grafton, S. T. (2021). Ventromedial Prefrontal Cortex Activity and Sympathetic Allostasis During Value-Based Ambivalence. Frontiers in Behavioral Neuroscience, 15. https://doi.org/10.3389/fnbeh.2021.615796
Hansen, J. O., Omland, P. M., Nilsen, K. B., Sand, T., & Matre, D. (2021). Experimental sleep restriction increases latency jitter in pain elicited cortical responses. Heliyon, 7(2). https://doi.org/10.1016/j.heliyon.2021.e06188
Hu, J., Wang, Z., Feng, X., Long, C., & Schiller, D. (2019). Post-retrieval oxytocin facilitates next day extinction of threat memory in humans. Psychopharmacology, 236(1), 293–301. https://doi.org/10.1007/s00213-018-5074-6
Le Mansec, Y., Dorel, S., Nordez, A., & Jubeau, M. (2019). Is reaction time altered by mental or physical exertion? European Journal of Applied Physiology. https://doi.org/10.1007/s00421-019-04124-7
Manaf, F. A., Peiffer, J. J., Maker, G. L., & Fairchild, T. J. (2021). Branched-chain amino acid supplementation improves cycling performance in untrained cyclists. Journal of Science and Medicine in Sport, 24(4), 412–417. https://doi.org/10.1016/j.jsams.2020.10.014
Mirabella, F., Desiato, G., Mancinelli, S., Fossati, G., Rasile, M., Morini, R., … Pozzi, D. (2020). Transient maternal IL-6 boosts glutamatergic synapses and disrupts hippocampal connectivity in the offspring. BioRxiv. https://doi.org/10.1101/2020.11.02.364356
Muhle, P., Labeit, B., Wollbrink, A., Claus, I., Warnecke, T., Wolters, C. H., … Suntrup-Krueger, S. (2021). Targeting the sensory feedback within the swallowing network—Reversing artificially induced pharyngolaryngeal hypesthesia by central and peripheral stimulation strategies. Human Brain Mapping, 42(2), 427–438. https://doi.org/10.1002/hbm.25233
Norum, M., Risvang, L. C., Bjørnsen, T., Dimitriou, L., Rønning, P. O., Bjørgen, M., & Raastad, T. (2020). Caffeine increases strength and power performance in resistance-trained females during early follicular phase. Scandinavian Journal of Medicine and Science in Sports, 30(11), 2116–2129. https://doi.org/10.1111/sms.13776
Oliva, M. K., Pérez-Moreno, J. J., O’Shaughnessy, J., Wardill, T. J., & O’Kane, C. J. (2020). Endoplasmic Reticulum Lumenal Indicators in Drosophila Reveal Effects of HSP-Related Mutations on Endoplasmic Reticulum Calcium Dynamics. Frontiers in Neuroscience, 14. https://doi.org/10.3389/fnins.2020.00816
Ree, A., Nilsen, K. B., Knardahl, S., Sand, T., & Matre, D. (2020). Sleep restriction does not potentiate nocebo-induced changes in pain and cortical potentials. European Journal of Pain (United Kingdom), 24(1), 110–121. https://doi.org/10.1002/ejp.1466
Shapiro, A. D., & Grafton, S. T. (2019). Subjective value then confidence in human ventromedial prefrontal cortex. BioRxiv. https://doi.org/10.1101/838276
Tomsen, N., Ortega, O., Rofes, L., Arreola, V., Martin, A., Mundet, L., & Clavé, P. (2019). Acute and subacute effects of oropharyngeal sensory stimulation with TRPV1 agonists in older patients with oropharyngeal dysphagia: a biomechanical and neurophysiological randomized pilot study. Therapeutic Advances in Gastroenterology, 12. https://doi.org/10.1177/1756284819842043