Product Focus
DS4 Bi-phasic Stimulus Isolator – Stimulate with arbitrary waveforms using your DAQ
External Control – Voltage In, Current Out
If you want greater control over stimulus shape than our DS2A or DS3 stimulators permit or a bi-phasic output, why not take a look at the DS4? The DS4 accepts a bi-phasic analogue “command” voltage and uses this waveform to define the shape of a bi-phasic constant current output, allowing DS4 users to deliver stimuli of any shape with external control of the stimulus parameters. This means that any user with a computer and data acquisition system (featuring an analogue output) can control their stimulation parameters from their acquisition software. The DS4 is recommended over our clinical DS5 stimulator for non-human and in vitro applications, especially those involving threshold tracking and nerve excitability studies using QtracW software.
Versatile – Wide Input and Output Ranges
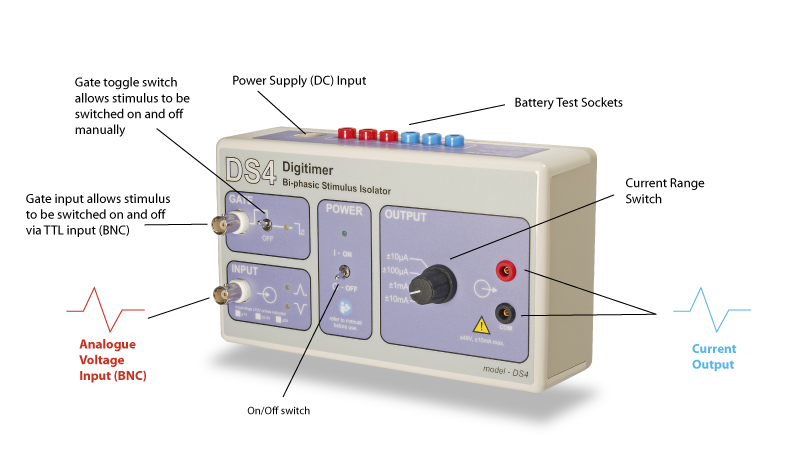
The DS4 accepts a variety of voltage input ranges (±1V, ±2.5V, ±5V and ±10V) and produces a constant current stimulus output in 4 overlapping ranges (±10µA, ±100µA, ±1mA and ±10mA) from a compliance voltage of ±48V. In addition, the DS4 has a GATE input which allows multiple DS4’s to be connected to a single analogue voltage source, with each DS4 being digitally enabled, separately.
Unique – Inactivity Sensor Reduces Leak Currents
One of the problems with stimulators that make use of an external voltage source to define a stimulus waveform is that small offsets or noisy baseline signals from the DAC’s used to drive them can result in unwanted battery drain, or perhaps worse, low amplitude stimulation. The DS4 uses a unique “inactivity sensor” to monitor the input voltage and disable the DS4 output if this voltage drops to 0±0.15% of the full scale value for a user selectable time period of 100ms, 200ms, 1s or 2s. Unlike other devices which only produce an output when the input voltage exceeds a threshold value, this “inactivity sensor” reduces battery usage and damaging “leak currents” during infrequent stimulation, while at the same time maintaining low levels of zero crossing distortion for repetitive waveforms.
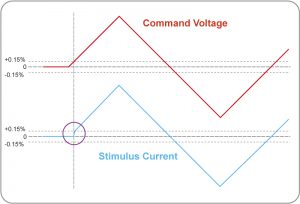
The figure below illustrates how the DS4 inactivity sensor “wakes up” the DS4 output in tens of microseconds, when the input voltage exceeds 0±0.15% of the full scale value, thereby initiating stimulation. The DS4 output remains active unless the input voltage drops below this threshold for a period longer than that set by the user selectable internal jumper. The inactivity sensor clamps the output current (blue) to zero until the command voltage (red) reaches a level greater than 0.15% of the full scale value, whereupon it rapidly increases the current to reach the requested level.
 Potential Applications for the DS4
Potential Applications for the DS4
The DS4 is ideally suited to applications that require low to medium amplitude constant current stimuli and where there may be a need for a biphasic, possibly arbitrary shaped waveform and/or external control of the stimulus parameters.
Example applications it has been employed in are:-
- Animal models of nerve excitability using threshold tracking techniques, under the control of QtracW software.
- Stimulation of isolated muscle and nerve preparations.
- In vivo peripheral nerve stimulation.
- Neuronal stimulation to study synaptic communication within whole animal or within brain slices.
- Stimulation of transmitter release in fast cyclic voltammetry.
- Simultaneous fMRI and deep brain stimulation.
- Galvanic vestibular stimulation in humans*
- Transcranial alternating current stimulation in studies of lucid dreaming** Note that the DS4 is not designed or approved for human use and we would only recommend our medically approved (EU MDD CE and US FDA 510(k)) DS5 stimulator for this.
Recent Publications
Moldovan, M., Pisciotta, C., Pareyson, D., & Krarup, C. (2020). Myelin protein zero gene dose dependent axonal ion-channel dysfunction in a family with Charcot-Marie-Tooth disease. Clinical Neurophysiology, 131(10), 2440–2451. https://doi.org/10.1016/j.clinph.2020.06.034
Develle, Y., & Leblond, H. (2020). Biphasic Effect of Buspirone on the H-Reflex in Acute Spinal Decerebrated Mice. Frontiers in Cellular Neuroscience, 13. https://doi.org/10.3389/fncel.2019.00573
Schreglmann, S. R., Wang, D., Peach, R. L., Li, J., Zhang, X., Latorre, A., … Grossman, N. (2020). Non-invasive Amelioration of Essential Tremor via Phase-Locked Disruption of its Temporal Coherence. BioRxiv. https://doi.org/10.1101/2020.06.23.165498
Wickham, R. J., Lehr, M., Mitchell, L., & Addy, N. A. (2020). Combined infusion and stimulation with fast-scan cyclic voltammetry (CIS-FSCV) to assess ventral tegmental area receptor regulation of phasic dopamine. Journal of Visualized Experiments. jove.com. https://doi.org/10.3791/60886
Lorentzen, J., Frisk, R., Willerslev-Olsen, M., Bouyer, L., Farmer, S. F., & Nielsen, J. B. (2020). Gait training facilitates push-off and improves gait symmetry in children with cerebral palsy. Human Movement Science, 69. https://doi.org/10.1016/j.humov.2019.102565
Blanchette-Carrière, C., Julien, S. H., Picard-Deland, C., Bouchard, M., Carrier, J., Paquette, T., & Nielsen, T. (2020). Attempted induction of signalled lucid dreaming by transcranial alternating current stimulation. Consciousness and Cognition, 83. https://doi.org/10.1016/j.concog.2020.102957
Austerschmidt, L. J., Khan, A., Plant, D. O., Richards, E. M. B., Knott, S., & Baker, M. D. (2020). The effects of temperature on the biophysical properties of optic nerve F-fibres. Scientific Reports. nature.com. https://doi.org/10.1038/s41598-020-69728-y
Willems, J. G. P., Wadman, W. J., & Cappaert, N. L. M. (2019). Interaction of cortical and amygdalar synaptic input modulates the window of opportunity for information processing in the rhinal cortices. ENeuro. ncbi.nlm.nih.gov. https://doi.org/10.1523/ENEURO.0020-19.2019
Kohli, S., & Casson, A. J. (2019). Removal of gross artifacts of transcranial alternating current stimulation in simultaneous EEG monitoring. Sensors (Switzerland), 19(1). https://doi.org/10.3390/s19010190
Willems, J. G. P., Wadman, W. J., & Cappaert, N. L. M. (2019). Excitation-Inhibition Dynamics Regulate Activity Transmission Through the Perirhinal–Entorhinal Network. Neuroscience. Elsevier. https://doi.org/10.1016/j.neuroscience.2019.05.031
Jeffrey-Gauthier, R., Piché, M., & Leblond, H. (2019). H-reflex disinhibition by lumbar muscle inflammation in a mouse model of spinal cord injury. Neuroscience Letters, 690, 36–41. https://doi.org/10.1016/j.neulet.2018.10.005
Sakae, D. Y., & Martin, S. J. (2019). Formation of a morphine-conditioned place preference does not change the size of evoked potentials in the ventral hippocampus–nucleus accumbens projection. Scientific Reports. nature.com. https://doi.org/10.1038/s41598-019-41568-5
Rakhilin, N., Garrett, A., Eom, C. Y., Chavez, K. R., Small, D. M., Daniel, A. R., … Shen, X. (2019). An intravital window to image the colon in real time. Nature Communications. nature.com. https://doi.org/10.1038/s41467-019-13699-w
Glover, I. S., & Baker, S. N. (2019). Multimodal stimuli modulate rapid visual responses during reaching. Journal of Neurophysiology, 122(5), 1894–1908. https://doi.org/10.1152/jn.00158.2019
De La Oliva, N., Del Valle, J., Delgado-Martinez, I., Mueller, M., Stieglitz, T., & Navarro, X. (2019). Long-Term Functionality of Transversal Intraneural Electrodes is Improved by Dexamethasone Treatment. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 27(3), 457–464. https://doi.org/10.1109/TNSRE.2019.2897256
Pastras, C. J., Curthoys, I. S., Sokolic, L., & Brown, D. J. (2018). Suppression of the vestibular short-latency evoked potential by electrical stimulation of the central vestibular system. Hearing Research, 361, 23–35. https://doi.org/10.1016/j.heares.2018.01.013
Laflamme, O. D., & Akay, T. (2018). Excitatory and inhibitory crossed reflex pathways in mice. Journal of Neurophysiology, 120(6), 2897–2907. https://doi.org/10.1152/jn.00450.2018
Wang, D., Tawfik, V. L., Corder, G., Low, S. A., François, A., Basbaum, A. I., & Scherrer, G. (2018). Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron. Elsevier. https://doi.org/10.1016/j.neuron.2018.03.002
Zaaimi, B., Dean, L. R., & Baker, S. N. (2018). Different contributions of primary motor cortex, reticular formation, and spinal cord to fractionated muscle activation. Journal of Neurophysiology, 119(1), 235–250. https://doi.org/10.1152/jn.00672.2017
Moldovan, M., Alvarez, S., Rothe, C., Andresen, T. L., Urquhart, A., Lange, K. H. W., & Krarup, C. (2018). An in Vivo Mouse Model to Investigate the Effect of Local Anesthetic Nanomedicines on Axonal Conduction and Excitability. Frontiers in Neuroscience. frontiersin.org. https://doi.org/10.3389/fnins.2018.00494
Willems, J. G. P., Wadman, W. J., & Cappaert, N. L. M. (2018). Parvalbumin interneuron mediated feedforward inhibition controls signal output in the deep layers of the perirhinal-entorhinal cortex. Hippocampus, 28(4), 281–296. https://doi.org/10.1002/hipo.22830
Fritz, B. M., Muñoz, B., Yin, F., Bauchle, C., & Atwood, B. K. (2018). A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience, 372, 1–15. https://doi.org/10.1016/j.neuroscience.2017.12.036
Jeffrey-Gauthier, R., Piché, M., & Leblond, H. (2017). Lumbar muscle inflammation alters spinally mediated locomotor recovery induced by training in a mouse model of complete spinal cord injury. Neuroscience, 359, 69–81. https://doi.org/10.1016/j.neuroscience.2017.07.010
Parent, K. L., Hill, D. F., Crown, L. M., Wiegand, J. P., Gies, K. F., Miller, M. A., … Cowen, S. L. (2017). Platform to Enable Combined Measurement of Dopamine and Neural Activity. Analytical Chemistry, 89(5), 2790–2799. https://doi.org/10.1021/acs.analchem.6b03642
François, A., Low, S. A., Sypek, E. I., Christensen, A. J., Sotoudeh, C., Beier, K. T., … Scherrer, G. (2017). A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron. Elsevier. https://doi.org/10.1016/j.neuron.2017.01.008
Arnold, R., Moldovan, M., Rosberg, M. R., Krishnan, A. V., Morris, R., & Krarup, C. (2017). Nerve excitability in the rat forelimb: a technique to improve translational utility. Journal of Neuroscience Methods, 275, 19–24. https://doi.org/10.1016/j.jneumeth.2016.10.013